Bahney Regenerative Therapeutics
2540 23rd Street,
Bldg 7, 3rd Floor, Rm 3110
San Francisco, CA 94110
Front Desk: 415-476-2124

In the Bahney Regenerative Therapeutics Laboratory, we are committed to developing innovative treatments and diagnostic tools that make a tangible difference in human health. Our work is rooted in the complex mechanisms behind normal tissue development and repair, providing us with the insights needed to address some of healthcare’s most pressing challenges.
We take a collaborative, interdisciplinary approach to our work, recognizing that solving complex clinical problems requires diverse perspectives and expertise. By fostering strong partnerships within the broader scientific community, we integrate insights from fields such as cell biology, bioengineering, and clinical medicine. Our multidisciplinary, team-oriented approach enables us to bridge the gap between fundamental scientific innovation and real-world application, driving the progress of effective therapeutic advancement to improve patient outcomes everywhere.
Our ultimate goal is to translate our research into practical, life-changing solutions—whether through new therapeutic strategies or cutting-edge diagnostic tools—that improve the quality of life for patients of all ages.
A complete list of the teams recent publications can be found here Google Scholar.
Fracture Pain Mechanisms and Therapeutic Targets
Fracture healing is a complex biological process that depends on inflammatory, neurological, and mechanical signaling to support tissue regeneration. Pain is one of the initial indicators of a bone fracture and serves both a functional and practical role in guiding recovery. However, fracture pain can persist long after the fracture itself has clinically healed, leading to impairments in mobility and quality of life. Our innovative research into fracture-related pain utilizes novel imaging tools for behavioral phenotyping in conjunction with evaluating the cellular and molecular mechanisms underlying fracture pain, with the goal of understanding the neuroimmune interactions that impact both pain modulation and bone regeneration. For this research, we leverage the power of artificial intelligence and machine learning to quantify biomechanical changes in movement and identify key pathways that contribute to acute and/or chronic pain states following fracture. Our research goal is to understand how regenerative treatments may decrease pain by accelerating healing and, conversely, look for strategies to mitigate pain without negatively impacting bone repair. In this new area of research for the Bahney Lab, we are excited to collaborate with pain experts Dr. Jarret Weinrich in the Department of Anesthesia and Allan Basbaum in Anatomy and Physiology
Publication Highlights
-
A bad break: mechanisms and assessment of acute and chronic pain after bone fracture
Nishimura H, Layne J, Yamaura K, Marcucio R, Morioka K, Basbaum AI, Weinrich JAP, Bahney CS. A bad break: mechanisms and assessment of acute and chronic pain after bone fracture. Pain. 2025 Nov 1;166(11):e491-e505. doi: 10.1097/j.pain.0000000000003646. Epub 2025 May 21. PMID: 40408239; PMCID: PMC12354150.
-
Deep behavioral phenotyping tracks functional recovery following tibia fracture in mice
JOUR, Layne, Jonathan E. ; Snapper, Dustin M. ; Czachor, Molly E. ; Lam, Charles ; Matityahu, Jacob D. ; Lind, Dane R. G. ; Huard, Matthieu; Huard, Johnny; Morioka, Kazuhito; Motzkin, Julian C.; Basbaum, Allan I.; Weinrich, Jarret A. P. ; Bahney, Chelsea S.
2025; Original Research; Deep behavioral phenotyping tracks functional recovery following tibia fracture in mice; Frontiers in Physiology; Volume 16 - 2025; 1664-042X
Stem Cell Reactivation
Our lab is very interested in the role of adult stem cells during tissue repair. Specifically work across multiple organs has found that reactivation of the master stem cell gene, Sox2, is critical to fracture repair and salivary gland function. We are interested in both understanding the molecular mechanisms that regulate stem cell activation and therapeutically reactivating this stem cell function to drive repair.
Publication Highlights
-
Losartan and Fisetin reduce senescence and enhance osteogenesis in human bone marrow derived mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine (Accepted)
Nishimura H, Yoichi Murata Y, Yamaura K, Mullen MM, O’Hara KM, Duke VR, Goff A, Huard C, Singer J, Nakayama N, Hambright WS, Ravuri S, Bahney CS, Philippon MJ, Huard J.
-

Long-term functional regeneration of irradiated salivary glands through delivery of a neurogenic hydrogel. Scientific Advances. 21 Dec 2022: Vol 8, Issue 51. *Co-Submitting
Long, J; Suiwala S; Berthoin L; May A; Mohabbat S; Gaylord E; Pacheco, N; Lombaert, I; Jeon O; Alsberg, E; Bahney CS*; Knox SM*.
-

Chondrocyte-to-Osteoblast Transformation in Mandibular Fracture Repair
Wong SA, Hu DP, Slocum J, Nguyen M, Miclau T, Marcucio RS, Bahney CS. Chondrocyte-to-Osteoblast Transformation in Mandibular Fracture Repair. J Orthop Res. 2021 Aug;39(8):1622-1632. PMID: 33140859 doi: 10.1002/jor.24904.
-

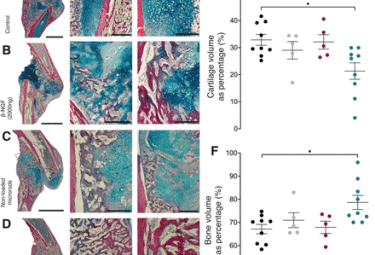
Chronic psychosocial stress disturbs bone fracture healing via β-adrenoceptor signaling
Haffner-Luntzer M, Foertsch S, Fischer V, Prystaz K, Tschaffon M, Mödinger Y, BahneyCS, Marcucio RS, Miclau T, Ignatius A. Chronic psychosocial stress disturbs bone fracture healing via β-adrenoceptor signaling. PNAS April 23, 2019 116 (17) 8615-862
-

Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes.
Hu DP, Ferro F, Yang F, Taylor AJ, Chang W, Miclau T, Marcucio RS, Bahney CS. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017 doi:10.1242/dev.130807
-

Stem cell derived endochondral cartilage stimulates bone healing by tissue transformation
Bahney CS, Hu DP, Taylor AJ, Ferro F, Britz, HM, Hallgrimsson B, Johnstone B, Miclau T, Marcucio RS. Stem cell derived endochondral cartilage stimulates bone healing by tissue transformation. Journal of Bone and Mineral Density (JBMR), 2013 DOI: 10.1002/jbmr.2148.
Tissue Engineering Strategies to Promote Tissue Regeneration
A foundation of our lab is developing novel products that could translate to the clinical and improve human health. We take a Developmental Engineering approach to designing bioactive or biomimetic platforms to promote tissue healing by studying the molecular and cellular mechanisms behind delayed healing (link to the molecular and cellular mech of delayed fracture repair) or loss of function. Biomaterials serve as the platform for drug and/or cell delivery in many of these systems, and we focus on optimizing the functional signal of the therapeutic, defining the appropriate therapeutic window, and promoting high quality tissue regeneration as opposed to repair through scarring.
Publication Highlights
-
β-catenin mRNA encapsulated in SM-102 lipid nanoparticles enhances bone formation in a murine tibia fracture repair model
Nelson AL, Mancino C, Choe JA, Chubb L, Williams K, Marcucio RS, Taraballi F, Cooke JP, Huard J, Bahney CS*, Ehrhart NE*. *Co-corresponding. β-catenin mRNA encapsulated in SM-102 lipid nanoparticles enhances bone formation in a murine tibia fracture repair model. Bioactive Biomaterials. 23 May 2024.
-
Localized delivery of β-NGF via injectable microrods accelerates endochondral fracture repair
Rivera KO, Cuylear DL, Duke V, O’Hara KM, Kharbikar BN, Kryger AN, Miclau T, Bahney CS, Desai TA. Localized delivery of β-NGF via injectable microrods accelerates endochondral fracture repair.
Frontiers in Bioengineering.
22 May 2023. -
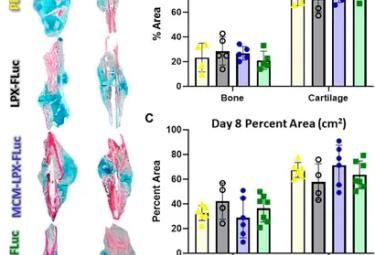
Mineral coated microparticles doped with fluoride and complexed with mRNA prolong transfection in fracture healing
Nelson AL, Fontana G, Chobb L, Choe J, Williams K, Regan D, Huard J, Murphy W, Ehrhart NE*, Bahney CS*. Mineral coated microparticles doped with fluoride and complexed with mRNA prolong transfection in fracture healing. *Co-corresponding. Frontiers in Bioengineering & Biotechnology. Volume 11 - 2023 | doi: 10.3389/ fbioe.2023.1295313
-
Therapeutic approaches to activate the canonical Wnt pathway for bone regeneration. Journal of Tissue Engineering and Regenerative Medicine
Nelson AL; Fontana G; Miclau E; Rongstand M; Murphy W; Huard J; Ehrhart NE; Bahney CS. Therapeutic approaches to activate the canonical Wnt pathway for bone regeneration. Journal of Tissue Engineering and Regenerative Medicine. 16 Sept 2022;
-
Long-term functional regeneration of irradiated salivary glands through delivery of a neurogenic hydrogel
Long, J; Suiwala; Berthoin; May; Mohabbat; Gaylord; Pacheco; Lombaert; Jeon; Alsberg, E; Bahney CS*; Knox SM*. Long-term functional regeneration of irradiated salivary glands through delivery of a neurogenic hydrogel. Scientific Advances. 21 Dec 2022: Vol 8, Issue 51. *Co-Submitting
-

Macromolecular crowding and decellularization techniques increase the growth factor binding potential of cell-secreted extracellular matrices
Fok SW; Gresham RCH; Ryan W; Osipov B; Bahney C, Leach JK. Macromolecular crowding and decellularization techniques increase the growth factor binding potential of cell-secreted extracellular matrices. Frontiers in Biomaterials and Biotechnology. Volume 11, 06 February 2023.
-
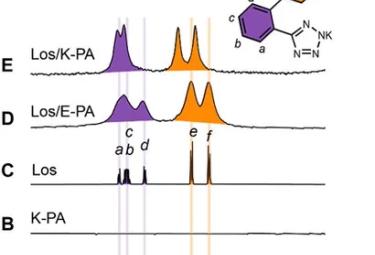
Sustained-release losartan from peptide nanofibers promotes chondrogenesis. Frontiers in Frontiers in Bioengineering and Biotechnology
Yamaura K; Sather NA; Metlushko A; Nishimura H; Pavlovic RZ; Hambright WS; Ravuri SK; Philippon MJ; Stupp SI; Bahney CS*; Huard J.* *Co-Submitting Sustained-release losartan from peptide nanofibers promotes chondrogenesis. Frontiers in Frontiers in Bioengineering and Biotechnology. Research Topic: Nanofibrous Biomaterials for Biomedical Applications. 06 Feb 2023.
-

A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells
Bahney CS, Hsu CW, Yoo JU, West JL, Johnstone B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 2011 May;25(5):1486-96. doi: 10.1096/fj.10-165514. Epub 2011 Jan 31. PMID: 21282205.
-
Promoting endochondral bone repair using osteoarthritic human articular chondrocytes
Bahney CS, Jacobs L, Tamai R, Hu DP, Wang M, Park M, Limburg S, Kim HT, Marcucio, RS, Kuo AC. Promoting endochondral bone repair using osteoarthritic human articular chondrocytes. Tissue Engineering Part A. 2016 Mar;22(5-6):427-35. doi: 10.1089/ten.TEA.2014.0705.
Biomarkers for Bone Healing
There is a significant clinical need for better tools to quantitatively measure bone fracture healing. The current gold standard relies on subjective physical exams and X-ray imaging to track tissue mineralization, but these methods are limited because most fractures heal through a cartilage phase before bone mineralization occurs. As a result, they can only assess later stages of healing, and diagnosing poorly healing fractures often requires 6 to 9 months of regular monitoring, or longer in elderly patients. Unfortunately, current technology is not sensitive enough to detect non-unions during the early stages of recovery.
Recent research by Drs Bahney and Working (OHSU, https://www.linkedin.com/in/zachary-working-5a19b4a9/) has shown that serum levels of collagen X (CXM), a protein associated with the cartilaginous phase of bone healing, serves as a reliable and sensitive biomarker for tracking fracture healing. Collagen X is produced by hypertrophic chondrocytes during the cartilage phase of bone repair, making it an especially useful marker for early-stage healing.
Our published and unpublished data show CXM levels differ between fractured and non-fractured patients, showing its potential to correlate with long-bone healing and other bone biomarkers. These findings suggest that CXM could significantly improve the ability to monitor fracture healing progress, particularly in the early stages.
We are continuing to enroll patients in clinical observation studies to validate CXM across a wide range of fracture types. This ongoing research aims to establish CXM as a reliable tool for assessing fracture healing in clinical settings and may lead to better, more timely interventions for patients with poor healing fractures.
Publication Highlights
-
Biomarker to Endochondral Phase of Fracture Repair Peaks Earlier than Traditional Markers and Detects Healing Differences in Long Bones
WorkingZM, Czachor ME, Nelson AL, O'Hara KM, Lancaster K, Coghlan R, Hellwinkel JE, Kaitlin Whitney K, Marchand LS, Bzovskys, DeKeyser GJ, Friess DM, Miclau T, Horton W, Sprague S, O'Hara NN, Johnstone B, Pierpoint LA, Slobogean G, on behalf of VitaShock Investigatorsà, Bahney CS. Biomarker to Endochondral Phase of Fracture Repair Peaks Earlier than Traditional Markers and Detects Healing Differences in Long Bones. Plos Medicine. (In Revision)
-
Collagen X Longitudinal Fracture Biomarker Suggests Staged Fixation in Tibial Plateau Fractures Delays Rate of Endochondral Repair
Working ZM, Peterson D, Lawson M, O’Hara KM, Coghlan RF, Provencher MT, Friess DM, Johnstone B, Miclau T, Bahney CS.
Collagen X Longitudinal Fracture Biomarker Suggests Staged Fixation in Tibial Plateau Fractures Delays Rate of Endochondral Repair. Journal of Orthopaedic Trauma (2022)
-
Bone turnover markers as surrogates of fracture healing after intramedullary fixation of tibia and femur
Stewart CS, O’Hara N, Bzovsky, Bahney CS, Sprauge S, Slobogean GP, Vita-Shock Investigators. Bone turnover markers as surrogates of fracture healing after intramedullary fixation of tibia and femur. Bone & Joint Research. 20 April 2022
-

A timeseries analysis of the fracture callus extracellular matrix proteome during bone fracture healing
Erickson CB, Hill R, Pascablo D, Kazakia G, Hansen K, Bahney CS. A timeseries analysis of the fracture callus extracellular matrix proteome during bone fracture healing. Journal of Life Sciences 2021 Dec;3(4):1-30. doi: 10.36069/JoLS/20220601.
-

Quantitative Serum Biomarker of Endochondral Ossification Effectively Correlates with Fracture Healing Progression
Working ZM, Morris ER, Chang JC, Coghlan RF, Schweitzer R, Miclau T, Horton W, Bahney CS. Quantitative Serum Biomarker of Endochondral Ossification Effectively Correlates with Fracture Healing Progression. Journal of Orthopaedic Research. 2020 Jun 13. PMID: 32533783
Molecular & Cellular Mechanisms of Delayed Fracture Repair
Bone fractures are one of the most common injuries worldwide with a significant global economic and social impact. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) completed a comprehensive, collaborative systematic analysis of the incidence and prevalence of 369 diseases and injuries across 204 countries and territories from 1990 to 2019. Specifically, the Fracture Collaborators reported 178 million new fractures, 445 million prevalent fractures, and 25.88 million years lived with disability in 2019. It is well-established that fracture repair is delayed at rates of up to 50% of fractures when they occur in situations of polytrauma or in patients with several common co-morbidities including age, diabetes, smoking, or obesity. The Bahney lab is interested in developing and characterizing preclinical systems that have delayed fracture healing. These models serve as the basis to identifying therapeutic targets to promote repair and platforms to validate novel treatments.
Publication Highlights
-
Murine Progeria Model Exhibits Delayed Fracture Healing with Dysregulated Local Immune Response
Duke VR, Philippon M Jr., Lind DRG, Kasler H, Yamaura K, Huard M, Czachor M, Hollenbeck J, Brown J, Garcia A, Fukase N, Marcucio RS, Nelson AL, Hambright WS, Snapper DS, Huard J, Bahney CS. Murine Progeria Model Exhibits Delayed Fracture Healing with Dysregulated Local Immune Response. Journal of Orthopaedic Translation (Submitted).
-
Polytrauma impairs fracture healing accompanied by increased persistence of innate inflammatory stimuli and reduced adaptive response
Saiz AM, Rahmati M, Gresham RCH, Baldini TD, Burgan J, Lee MA, Osipov B, Christiansen BA, Khassawna TE, Wieland DCF, Marinho AL, Blanchet C, Czachor M, Working ZM, Bahney CS, Leach JK. Polytrauma impairs fracture healing accompanied by increased persistence of innate inflammatory stimuli and reduced adaptive response. Journal of orthopaedic research. 2024. doi: 10.1002/jor.26015. PubMed PMID: 39550711.
-

Altered early immune response after fracture and traumatic brain injury
Haffner-Lutzer M; Weber B; Morioka K; Lackner I; Fischer V; Bahney CS; Ignatius A; Kalbitz M; Marcucio RS; Miclau T. Altered early immune response after fracture and traumatic brain injury. Frontiers in Immunology. 2023 Jan 25;14:1074207. PMID: 36761764
-
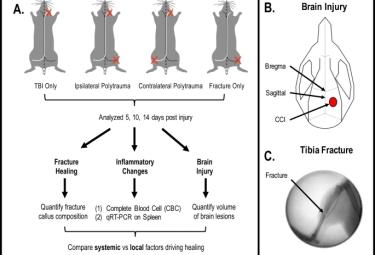
Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma
Morioka, K. Marmor Y, Sacramento JA, Amity L, Shao T, Miclau KR, Clark D, Beatie MS, Marcucio RS, Miclau T, Ferguson AR, Bresnahan JC, Bahney CS. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Scientific Reports 9, 12199, doi:10.1038/s41598-019-48126-z (2019).
-

Chronic psychosocial stress disturbs bone fracture healing via β-adrenoceptor signaling
Haffner-Luntzer M, Foertsch S, Fischer V, Prystaz K, Tschaffon M, Mödinger Y, Bahney CS, Marcucio RS, Miclau T, Ignatius A. Chronic psychosocial stress disturbs bone fracture healing via β-adrenoceptor signaling. PNAS. DOI: 10.1073/pnas.1819218116, PMID: 30948630, PMC6486758
-
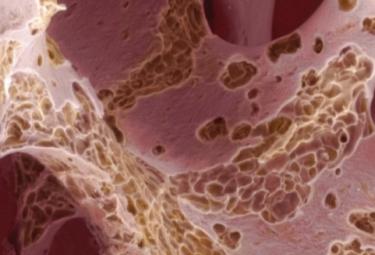
The senolytic drug fisetin attenuates bone degeneration in the Zmpste24-/- progeria mouse model
Hambright WS, Mu X, Kawakami Y, Mitchell J, Mullen M, Nelson AL, Bahney CS, Gao X, Hellwinkel J, Eck A, Huard J. The senolytic drug fisetin attenuates bone degeneration in the Zmpste24-/- progeria mouse model. Journal of Osteoporosis. 22 Feb 2023
Research Facilities at UCSF
-
Surgical Training Facility (STF)
The STF Lab offers top-of-the-line technology with the most comprehensive advanced imaging of any academic surgical training facility in the United States. Click here for detailed information.
-
Biomechanical Testing Facility
The BTF is part of the Orthopaedic Trauma Institute at the University of California, San Francisco, Orthopedic Department, housed in Zuckerberg San Francisco General Hospital (ZSFG). Click here for detailed information.
-
UCSF Pride Hall
Click here for location and detailed information.