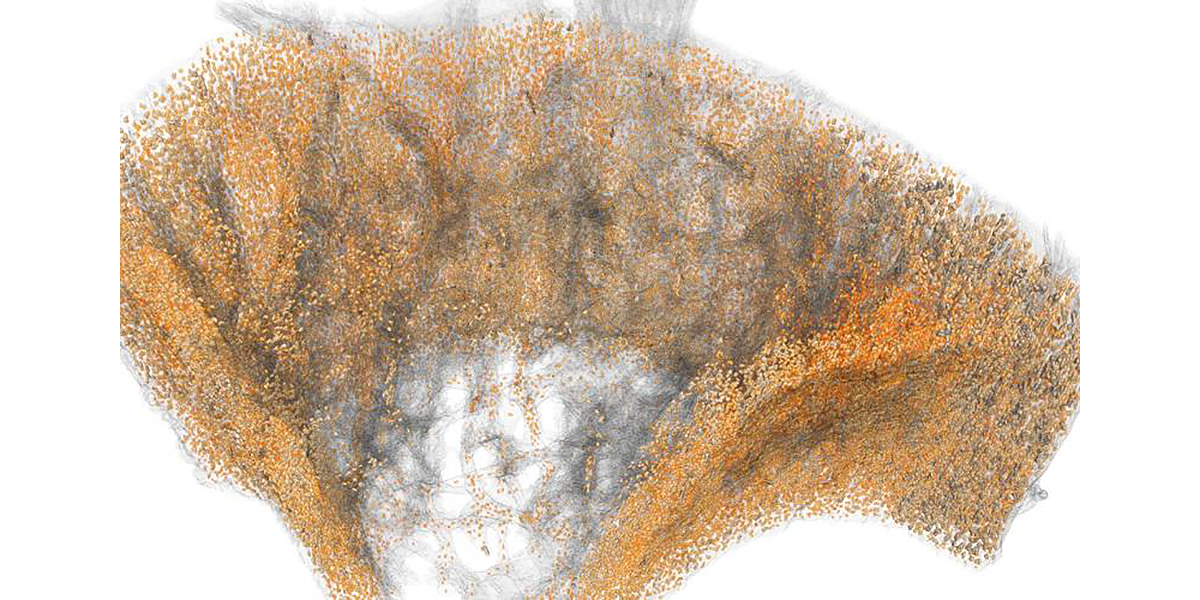
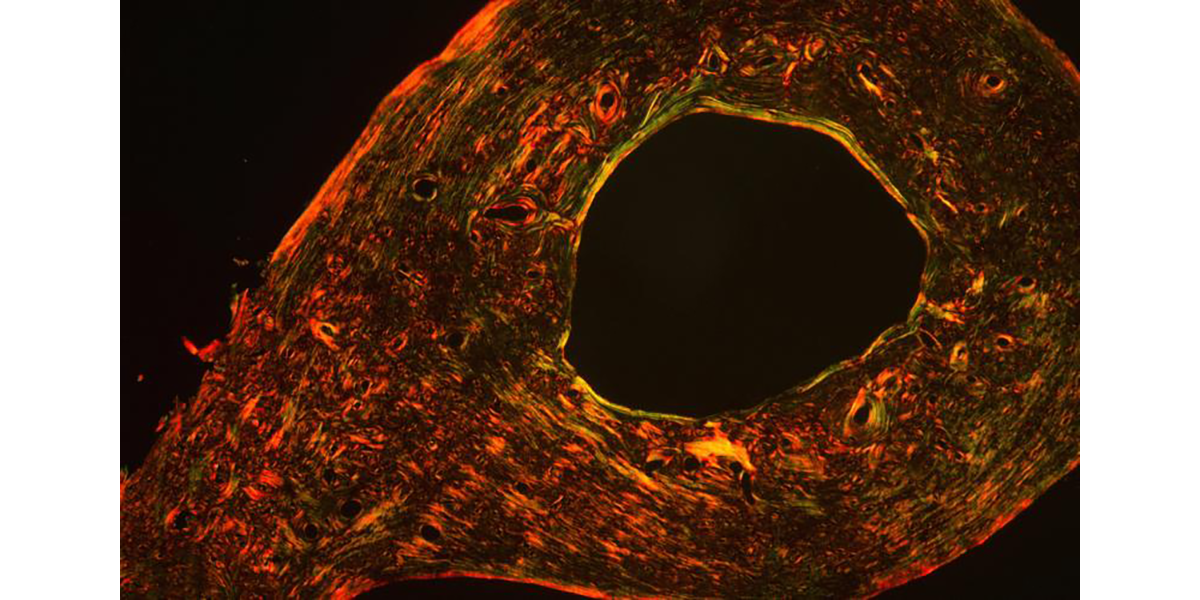
Alliston Laboratory for Skeletal Cell Differentiation and Signaling
513 Parnassus Ave. S-1164
San Francisco, CA 94143
Telephone: 415-502-4945
Our work
Tamara Alliston, PhD, and her lab are focused on the molecular pathways controlling mesenchymal stem cell differentiation, how these pathways coordinate with physical cues to influence mechanical integrity of normal skeletal tissue, and how they can be harnessed to repair tissue damaged in degenerative skeletal disease. In particular we focus on defining the function of TGFβ in synergistically coordinating physical and biochemical cues to regulate skeletal cell differentiation. To answer these questions we combine molecular, cellular, physiologic, and materials science approaches. This interdisciplinary approach will lead to the identification of targets to prevent skeletal disease or to improve skeletal repair. For more information, please visit the Alliston Lab web site.

Alliston Lab at UCSF
Current projects
Topics covered in our research include:
Mechanisms of TGFβ Action in Bone
TGFβ is a powerful regulator of homeostasis in skeletal cells and tissues. Dr. Alliston’s early work identified a transcriptional mechanism by which TGFβ inhibits osteoblast differentiation. Specifically, the TGFβ effector Smad3 recruits histone deacteylases to repress transactivation by the osteogenic transcription factor Runx2. This work provided a foundation for understanding mechanisms by which TGFβ regulates bone and cartilage development and homeostasis. In the clinical realm, we were the first to demonstrate the beneficial effects of pharmacologic TGFβ inhibition on bone mass and quality, which result from anabolic and anticatabolic effects. Similar molecules are currently in clinical trials for the treatment of bone fragility in osteogenesis imperfecta.
Biological Control of Bone Quality
Like bone mass, the material quality of bone is carefully regulated and functionally essential for maintaining resistance to fracture. We found that the same mechanisms by which growth factors and transcription factors direct skeletal cell differentiation also specify key physical properties of the extracellular matrix (ECM), the first biological pathways shown to do so. Specifically, we showed that Runx2 specifies material properties of bone ECM in a TGFβ-dependent manner. Deregulation causes hearing loss associated with cleidocranial dysplasia and likely other bone syndromes. We have applied our expertise to examine several other factors that regulate bone quality, studies that suggest new cellular and molecular targets for the development of therapies to prevent bone fragility.
Osteocyte-mediated Perilacunar Remodeling
Osteocytes play a critical role in bone maintainence by directing the activity of osteoblasts and osteoclasts to regulate bone mass, and by remodeling the perilacunar ECM to maintain bone quality. Our work has revealed that MMP13 is essential for osteocyte-mediated remodeling of the perilacunar bone ECM. Relatively little is known about the molecular regulation or role of this emerging cellular mechanism, perilacunar remodeling, in the maintenance of bone health or disease. This research may elucidate novel therapeutic targets to prevent the loss of bone quality in a variety of skeletal diseases, including osteoporosis and osteonecrosis.
Skeletal Mechanobiology
Because ECM elastic modulus drives changes in cellular tension that direct cell differentiation, we investigated the effect of cellular tension on chondrocyte differentiation. We use a multi-scale approach, from the organism to the single-molecule level, to investigate the underlying mechanisms by which cells integrate physical and biochemical cues in bone and cartilage. We found that chondrocyte cellular tension primes cells for a robust response to TGFβ. Specifically, we showed for the first time that TGFβ receptors take on a tension-sensitive spatial localization that modulates their responsiveness to TGFβ ligand. This work will elucidate mechanisms by which physical cues control TGFβ signaling, and potentially those that contribute to the loss of chondrocyte homeostasis in osteoarthritis, in which the material quality of cartilage ECM progressively deteriorates.

Alliston Laboratory for Skeletal Cell Differentiation and Signaling
University of California, San Francisco
513 Parnassus Ave., S-1156
San Francisco, CA 94143