international Center for Osseointegration Research, Education and Surgery (iCORES)
Our work
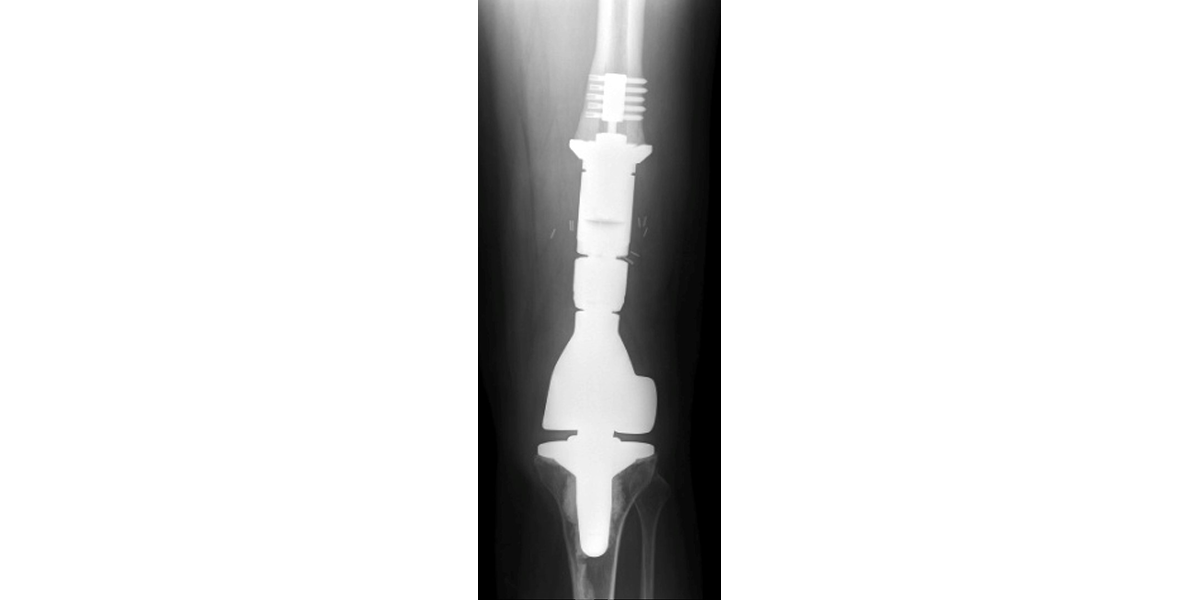
Part of the UCSF’s Orthopaedic Oncology Service, the international Center for Osseointegration Research, Education and Surgery (iCORES) program focuses on basic, clinical, and translational research involving bone-anchored implants. Historically involved in the utilization of the Compress® device for fixation of endoprostheses used in the reconstruction of massive skeletal defects in cancer patients, we are now investigating osseointegration technology to help in the rehabilitation of patients with amputation. Advanced studies include efforts to develop brain-machine interface systems that enable bidirectional intuitive volitional control of, and sensory/proprioceptive feedback from, external prostheses. Richard J. O’Donnell, M.D. serves as Director of the iCORES program. He is assisted by Drs. Rosanna L. Wustrack and Melissa N. Zimel.
Current projects
iCORES currently has a total of 12 research protocols approved by the UCSF Institutional Review Board (IRB), including 4 clinical trials for patients with upper and/or lower extremity amputations who may be candidates for the use of osseointegration technology to improve rehabilitation potential. Interested individuals can contact our program at iCORESinfo@ucsf.edu. Our research is funded in part through four federal contracts and grants. We are thankful for the support of colleagues and funders at the Department of Defense Osseointegration Program; the Walter Reed National Military Medical Center; the Uniformed Services University; the Defense Advanced Research Projects Agency (DARPA); the U.S. Army Medical Research and Development Command (USAMRDC); the Congressionally Directed Medical Research Program (CDMRP); the Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF); the Massachusetts Institute of Technology; Harvard Medical School; the Brigham and Women’s Hospital; Chalmers University of Technology; the Johns Hopkins University; and the University of California, Berkeley.
IRB approved research protocols
- 18-25115 Neuroimaging-Based Objective Diagnostic Tool to Detect Subjective Tinnitus
- 21-33990 OPRA Implant System Premarket Approval and Related Studies
- 20-32576 A 2-year follow-up, multidisciplinary study focused on outcomes and experience with the OPRA Axor II coupling unit
- 20-31453 Phantom limb pain multimodal imaging
- 20-30167 Osseointegration Quality Registry
- 19-28003 ASSIST: Amputee Safe Smart Implant Sensor Technology
- 18-25641 Transfemoral Amputation Osseointegration Study, TFAOS, Non-Research, HUD Only
- 18-25404 Transfemoral Amputation Osseointegration Study, TFAOS, Research
- 18-25115 Neuroimaging-Based Objective Diagnostic Tool to Detect Subjective Tinnitus
- 18-24966 Transhumeral e-OPRA, An osseoanchored percutaneous prosthesis study evaluating stable neural signal transmission in subjects with transhumeral amputations
- 18-24855 Incidence of amputees who may be candidates for an osseointegrated percutaneous prosthetic system
- 17-22205 Patient Preferences for Attributes of Risk and Benefit of Prosthetic Devices for Upper Limb Loss
- 17-21928 An osseointegrated transfemoral prosthesis offering long-term bidirectional efferent-afferent neural transmission (IDE)
- 16-19786 Transhumeral Amputation Osseointegration Study, TAOS
- 15-18025 Compressive osseointegration for distal femur reconstruction in giant cell tumor
- 15-16764 Osseointegrated percutaneous prosthetic system for transfemoral amputees; A prospective cohort study
- 10-02939 Reconstruction of massive defects using the ComPreSs device for endoprosthetic fixation in the proximal femur, distal femur, proximal tibia and humerus
- DARPA-W911NF-17-2-0043. An osseointegrated transfemoral prosthesis offering long-term bi-directional efferent-afferent neural transmission.
- W81XWH-17-2-0060, USAMRMC-BA160465. Transfemoral Amputee Osseointegration Study (TFAOS).
- W81XWH-17R-BAA1. An osseo-neural transtibial prosthesis with efferent-afferent neural control.
- W81XWH-19-OPORP-CRA. Assessing biomechanical function and hip stabilizing muscle quality associated with transfemoral osseointegration.
Publications
- Zaid, M.B.; O'Donnell, R.J.; Potter, B.K.; and Forsberg, J.A.: Orthopaedic osseointegration: State of the art. J.Amer. Acad. Orthop. Surg., 27(22):e977-e985, 2019. doi: 10.5435/JAAOS-D-19-00016. PubMed PMID: 31181031.
- Zaid, M.B.; Wustrack, R.L.; Garibaldi, M.; Geiger, E.; Andaya, V.; and O’Donnell, R.J. Prospective study of percutaneous bone-anchored implants in transfemoral amputees: Brain-machine platform technology for external prosthetic control and feedback. Conf. Proc. IEEE Eng. Med. Biol. Soc., 2019 Mar;2019:13-16.
- Strony, J.; Brown, S.; Choong, P.; Ghert, M.; Jeys, L.; and O’Donnell, R.J.: Musculoskeletal infection in orthopaedic oncology: Assessment of the 2018 International Consensus Meeting on Musculoskeletal Infection. J. Bone Joint Surg., 101-A(20):e107, 2019. doi: 10.2106/JBJS.19.00182. PubMed PMID: 31626015.
- Goldman, L.H.; Morse, L.J.; O’Donnell, R.J.; and Wustrack, R.L. How often does spindle failure occur in compressive osseointegration endoprostheses for oncologic reconstruction? Clin. Orthop. Rel. Res., 474:1714-1723, 2016. doi: 10.1007/s11999-016-4839-7. Epub 2016 Apr 22. PubMed PMID: 27106130.
- Legaspi, K.N.; Wustrack, R.L.; and O’Donnell, R.J.: Total femoral and proximal tibial compressive osseointegration: 12-year follow-up. Curr. Orthop. Pract., 27(2):231-235, 2016. doi: 10.1097/BCO.0000000000000334.
- Pedtke, A.C.; Wustrack, R.L.; Fang, A.S.; Grimer, R.J.; and O’Donnell, R.J.: Aseptic failure: How does the Compress® implant compare to cemented stems? Clin. Orthop. Rel. Res., 470:735-742, 2012. doi: 10.1007/s11999-011-2159-5. Epub 2011 Nov 2. PubMed PMID: 22045069.
- O’Donnell, R.J.: Compressive osseointegration of tibial implants in primary cancer reconstruction. Clin. Orthop. Rel. Res., 467:2807-2812, 2009. doi: 10.1007/s11999-009-0986-4. Epub 2009 Aug 4. PubMed PMID: 19653050.
- Tyler, W.K.; Healey, J.H.; Morris, C.D.; Boland, P.J.; and O’Donnell, R.J.: Compress® periprosthetic fractures: Interface stability and ease of revision. Clin. Orthop. Rel. Res., 467: 2800-2806, 2009. doi: 10.1007/s11999-009-0946-z. Epub 2009 Jun 30. PubMed PMID: 19565305.
- Kramer, M.J.; Tanner, B.J.; Horvai, A.E.; and O’Donnell, R.J.: Compressive osseointegration promotes viable bone at the endoprosthetic interface: Retrieval study of Compress® implants. Intl. Orthop., 32:567-571, 2008. doi: 10.1007/s00264-007-0392-z. Epub 2007 Jun 19. PubMed PMID: 17576554.
- O’Donnell, R.J.: Compressive osseointegration of modular endoprostheses. Current Opinion Orthop., 18:590-603, 2007. doi: 10.1097/BCO.0b013e3282f0dafc.
- Avedian, R.S.; Goldsby, R.E.; Kramer, M.J.; and O’Donnell, R.J.: Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin. Orthop. Rel. Res., 459:48-53, 2007. doi: 10.1097/BLO.0b013e3180514c66. PubMed PMID: 17545758.
- Bhangu, A.A.; Kramer, M.J.; Grimer, R.J.; and O’Donnell, R.J.: Early distal femoral endoprosthetic survival: Cemented stems versus the Compress® implant. Intl. Orthop. 30:465-472, 2006. doi: 10.1007/s00264-006-0186-8. Epub 2006 Sep 16. PubMed PMID: 16983554.